What is COVID-19
COVID-19, the latest global pandemic, is a coronavirus disease that causes respiratory illness that can spread quickly from human to human. The latest outbreak of COVID-19 caused about 7,000 confirmed cases in China in the first month (January 2020, Situation report), following with another 80,000 confirmed worldwide in the second month (February 2020, Situation report).
Difference between COVID-19, MERS and SARS
Coronaviruses are a wide family of viruses that cause disease, often in animals. However, 7 forms of coronaviruses can cause disease in humans, and 3 of these can cause significant outbreaks of deadly pneumonia:
- COVID-19 – It is an infectious disease caused by the most recently identified coronaviruses. The “SARS-CoV-2 virus” is a novel coronavirus that was first identified in Wuhan, China at the end of 2019 as a disease with extreme acute respiratory syndrome that actively spread worldwide in 2020.
- MERS – The Middle East respiratory syndrome is a viral respiratory disease caused by coronavirus. The virus “MERS-CoV” was first identified in Saudi Arabia in 2012.
- SARS – The virus “SARS-CoV” was reported in 2002 as the cause of an outbreak of severe acute respiratory syndrome (SARS).
According to figures from the World Health Organization (WHO), SARS and MERS took years to spread and killed more than 800 people. Yet COVID-19 took just 3 months to spread around the world, causing about 115,000 deaths worldwide. Such high numbers that have occurred in a short time can cause the local medical resource to crash.
How to ensure protection level sufficient for healthcare personnel
According to WHO guidelines, the virus can spread directly when a case of COVID-19 coughs or exhales droplets that touch the nose, mouth or eyes of another person. Keep your hands clean and cover your mouth and nose while coughing or sneezing is vital for public health. Yet it’s another matter for healthcare services.
In order to prevent mass infection in healthcare facilities, infected patients need to remain in a controlled environment, namely negative pressure isolation room. Frontline operators need to wear a complete range of isolation equipment, like face shield, N95 respirator, coverall, gloves, boot covers, etc. according to WHO Disease Commodity Packages (DCPs) of COVID-19, or CDC Coronavirus Disease Infection Control.
Is it enough if wear all the equipment suggested? Typically, environmental variables are under-controlled in a healthcare facility. Normally, biological threats come from a few directions, usually from the lower front, since the patient can sit or lie down on the bed. Protective equipment used in the medical industry, such as isolation gown, is intended to avoid front contamination only. But the region above or below the chest is exposed and can cause possible hazards. Once it comes to coronavirus disease, such as COVID-19, protection for the human body is not enough.
In order to get an appropriate protection, coverall is a safer choice when dealing with disease prevention, such as COVID-19. Coverall has a one-piece hood, gloves, body cover and pants that eliminates all gaps in the collar, chest, legs and back section. These will have 360 degrees of protection for healthcare personnel.
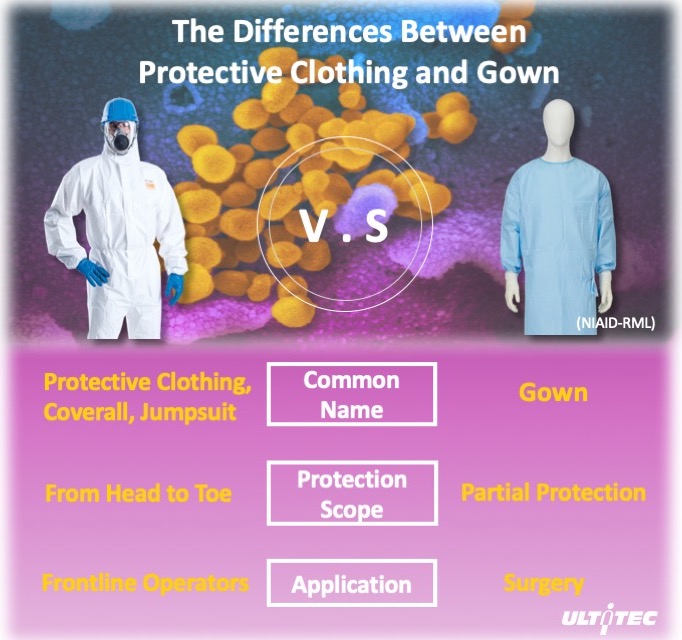
Figure 1. Differences between coverall and isolation gown
How does EN 14126 provides protection
The World Health Organization (WHO) has recently warned that COVID-19 is a “very high” risk pandemic to be taken seriously. In order to prevent misuse of Personal Protective Equipment (PPE) during this battle, it is time to take a closer look at EN 14126, which provides specifications and test methods for evaluating the protection of the fabric against infective agents.
According to EN 14126, special requirements are defined for protective clothing against infectious agents to protect against bacteria, viruses and other microorganisms. It contains 5 different tests, especially 3 of them are key indexes to protect against COVID-19, which are ISO 16603, ISO 16604, and ISO/DIS 22611 which determine penetration by blood, body fluids, blood-borne pathogens and biologically contaminated liquid aerosol.
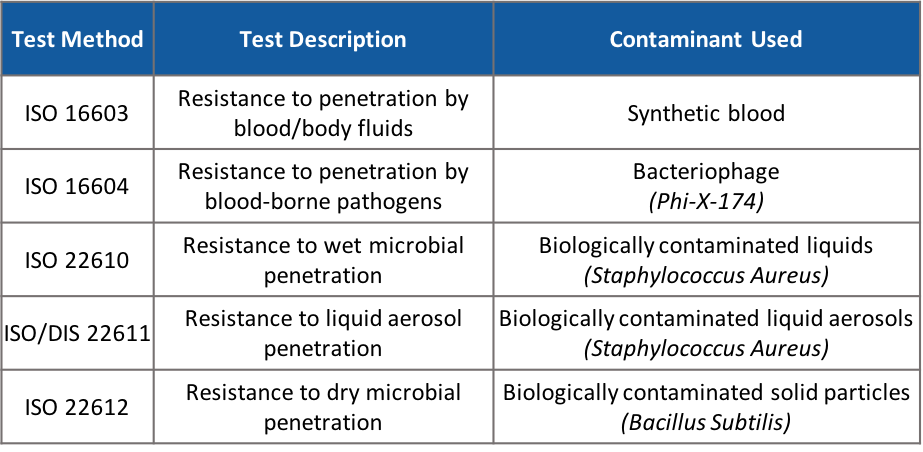
Figure 2. Test methods required under EN 14126
ISO 16603 test is focus on the penetration ability under certain pressure with synthetic blood. It can simulate the contamination from infected blood or body fluids contact on your clothing. The higher class present the higher protection level for a protective clothing.
The size of the COVID-19 virus is approximately 0.125 microns. From the above contaminants, Phi-X-174 (0.027 microns) is the only contaminant which smaller than COVID-19. Hence, if the protective clothing passes ISO 16604 with a relatively high protection class, it means that it has a higher protection level.
If you are a frontline healthcare personnel, you may want to concentrate on ISO/DIS 22611. Earlier in February, China acknowledged the possibility of aerosol transmission. This may happen when the patient sneezes hard or when you have been exposed to large concentrations of aerosol in a confined space for a long time, such as inserting a respiratory tube during medical procedures, which triggers a burst of mass aerosols.
Summary
Although WHO and CDC had full guidance of selecting proper PPE against COVID-19, but the instructions does not include coveralls. If coping with diseases that require effective infection prevention, we recommend that healthcare personnel should not be limited to medical equipment, coverall is the appropriate option and should be prepared in each medical facility to avoid another outbreak of disease.
Please also note to test your protective clothing if it has EN 14126 certification. EN 14126 is a material test that proves that the fabric has a barrier to biological disease. In the global case of COVID-19, certified protective clothing EN 14126 can effectively prevent the transmission of disease along with other PPE if appropriate selection is made.
- ULTITEC Played Significant Role During COVID-19
- Understanding EN 14126:2003 for Battle Against COVID-19
- What Protective Clothing Is Appropriate for Novel Coronavirus (COVID-19) Disease Control ?
- What should you understand about CE test standard for protective clothing?
- How Well Do You Understand EN 14126:2003? Protection Against Biological Agents in Workplace
- COVID-19: What Can You Do to Protect Yourself
- EN 14126 Certified Protective Clothing to Protect Against COVID-19

